As Seen In
Stem cell ‘Wild West’ takes root amid lack of US regulation


Business | By Matthew Perrone | AP May 18, 2015
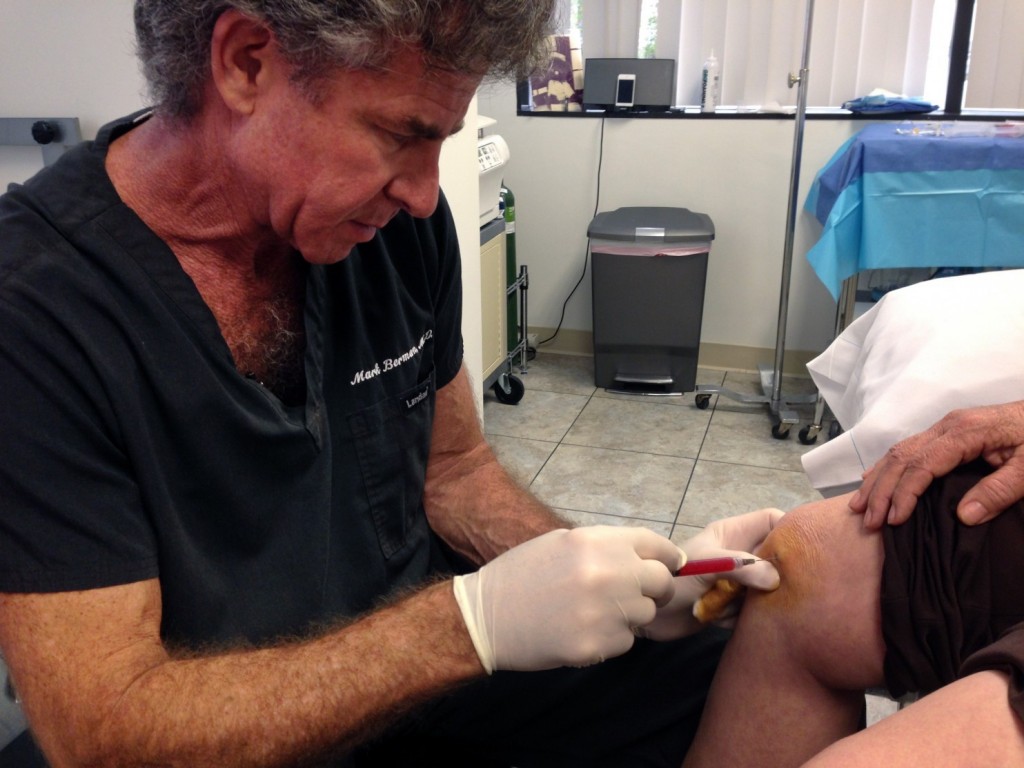
In this Dec. 5, 2014 photo, Dr. Mark Berman, of the Cell Surgical Network, injects a patient with a solution he says is rich in adult stem cells, at his practice in Beverly Hills, Calif. Berman’s company is the largest in the mushrooming industry of for-profit clinics that market stem cells to patients with dozens of different diseases and conditions. Critics say the businesses have flourished due to a lack of government oversight. (Raquel Dillion/Associated Press)
BEVERLY HILLS, Calif. — The liquid is dark red, a mixture of fat and blood, and Dr. Mark Berman pumps it out of the patient’s backside. He treats it with a chemical, runs it through a processor — and injects it into the woman’s aching knees and elbows.
The “soup,” he says, is rich in shape-shifting stem cells — magic bullets that, according to some doctors, can be used to treat everything from Parkinson’s disease to asthma to this patient’s chronic osteoarthritis.
“I don’t even know what’s in the soup,” says Berman. “Most of the time, if stem cells are in the soup, then the patient’s got a good chance of getting better.”
It’s quackery, critics say. But it’s also a mushrooming business — and almost wholly unregulated.
The number of stem-cell clinics across the United States has surged from a handful in 2010 to more than 170 today, according to figures compiled by The Associated Press. Many of the clinics are linked in large, for-profit chains. New businesses continue to open; doctors looking to get into the field need only take a weekend seminar offered by a training company.
Berman, a Beverly Hills plastic surgeon, is co-founder of the largest chain, the Cell Surgical Network. Like most doctors in the field, he has no formal background in stem cell research. His company offers stem cell procedures for more than 30 diseases and conditions, including Lou Gehrig’s disease, multiple sclerosis, lupus and erectile dysfunction.
There are clinics that market “anti-aging” treatments; others specialize in “stem-cell facelifts” and other cosmetic procedures. The cost is high, ranging from $5,000 to $20,000.
Berman and others point to anecdotal accounts of seemingly miraculous recoveries. But while stem cells from bone marrow have become an established therapy for a handful of blood cancers — and while there are high hopes that the cells will someday lead to other major medical advances — critics say entrepreneurs are treating patients with little or no evidence that what they do is effective.
Or even safe. They point to one stem-cell doctor who has had two patients die under his care.
“It’s sort of this 21st century cutting-edge technology,” says Dr. Paul Knoepfler, a stem cell researcher at the University of California at Davis. “But the way it’s being implemented at these clinics and how it’s regulated is more like the 19th century. It’s a Wild West.”
DISCOVERING ‘LIQUID GOLD’
Doctors in South Korea and Japan pioneered the fat-based stem cell technique, using it to supposedly enhance face lifts and breast augmentation. For years, U.S. patients would travel to hospitals in Asia, Latin America and Eastern Europe — places where regulation is more lax than in the United States — to have these procedures as part of the international “stem cell tourism” trade.
Plastic surgeons in the U.S. quickly realized the financial potential of the fat they were already taking out of patients’ bellies and backsides through liposuction — something that had been disposed of previously. Berman calls it “liquid gold.”
Some early adopters have expanded into chains, offering doctors across the country a chance to join the franchise after buying some equipment and attending a seminar. These doctors sometimes appear on local TV news broadcasts, drumming up new business from patients and stoking interest from other doctors.
One national chain markets itself online with accounts of celebrity athletes who have been treated with its stem cell procedures. Prospective patients are then directed to a call center, where sales representatives try to match them with stem cell doctors over the phone.
Berman spent over 30 years as a Beverly Hills cosmetic surgeon before co-founding the Cell Surgical Network in 2012. He and his business partner, a urologist, adapted equipment and techniques from Asia into a liposuction-based procedure.
Today, the Cell Surgical Network is the largest stem cell chain in the nation, with 67 locations and a roster of more than 100 doctors in 22 states. Doctors who join the network generally charge about $9,000 per procedure; they pay Berman and his partner $25,000 to $30,000 for a South Korean cell-separating machine and other equipment.
Stem cells have long been recognized for their ability to reproduce and transform into other cell types. Because of their ability to repair and replace tissue, they are thought to hold potential for treating many diseases and injuries.
Embryonic stem cells are the most versatile because they have the ability to form all the various cell types in the body, but their use in medicine is considered controversial by some because it involves the destruction of human embryos.
Adult stem cells are less versatile, but can be easily harvested from various tissues in the body, including bone marrow and fat. For decades, they have been routinely transplanted, first in bone marrow transplants and then in procedures that transfer the cells alone. They have been useful in combatting leukemia, lymphoma and other blood diseases, saving the lives of tens of thousands of people each year.
The stem cell clinics, though, promise results far beyond those currently considered prudent by mainstream medicine.
“I think responsible professionals have a broad consensus that marketing of these unproven interventions is premature and unprofessional, if not unethical,” says Dr. George Daley, a founding executive of the Harvard Stem Cell Institute and professor at Harvard Medical School
Julia Matsumoto, of Fountain Valley, Calif., claims stem cell injections have helped maintain her eyesight four years after being diagnosed with chronic relapsing neuropathy, which causes inflammation of the optic nerves and can lead to blindness.
Berman has treated her on a monthly basis since 2012, free of charge, because Matsumoto cannot afford repeat procedures. Berman liposuctions fat from her abdomen then processes it with a spinning centrifuge machine and a drug, before filtering it and infusing the mixture into an injection site in Matsumoto’s chest.
“Things were so vivid and bright literally 30 minutes after the stem cells were given to me,” Matsumoto says, recalling her first treatment. “I started crying on the way home.”
Such patient anecdotes are not considered reliable medical evidence. And because stem cell clinics have not published large, rigorous studies of their techniques, it’s virtually impossible to evaluate their record of success.
Berman calls his business model “patient-funded research,” and says he plans to soon publish the results of a 1,000-patient study demonstrating its safety. Cell Surgical has hired consultants to follow up with patients over the phone and survey how they are feeling.
But Leigh Turner, a professor of bioethics at the University of Minnesota, says charging patients to participate in medical research is bizarre and unethical. He calls the approach “unauthorized, for-profit human experimentation,” and has asked the Food and Drug Administration to investigate Berman, arguing that his business amounts to selling unapproved, experimental drugs.
Some practitioners point to early-stage laboratory and animal studies which have been published in scientific journals. But academics say such findings cannot be applied to humans and don’t provide critical information about potential side effects like infections, tumors and blood clots.
“This field, sadly, is contaminated by lots of poor-quality data that people are using to move forward and actually treat patients,” says Daley, of Harvard Medical School.
THE RISKS
The clinics insist that their treatments are safe, but routinely require that patients sign waivers.
Cell Surgical’s patients sign an informed consent form acknowledging that they are participating in an experimental study. The form states that there is no guarantee that the stem cell treatment will work, and lists potential risks. It also makes clear that patients are responsible for paying the full cost of the procedure, which is not covered by insurance.
Patients of Dr. Zannos Grekos, a cardiologist in Bonita Springs, Florida, who specialized in using stem cells to treat debilitating diseases, also were required to sign a consent form, acknowledging the procedures’ risks, including death.
But families of at least two of Grekos’ patients say he downplayed the risks. Gina Adams, daughter of patient Richard Poling, says her family was told her father would be “back on the golf course the next day” after a routine procedure he hoped would help him recover from a lung condition that made breathing difficult. The cost was $8,000.
The family was told that the stem cells would regenerate Poling’s lung cells.
In March 2012, Grekos harvested fat from Poling’s abdomen and sent it to an off-site processing facility to isolate the stem cells. Later that afternoon, he directed an assistant to infuse the resulting mixture into the patient’s bloodstream.
Poling suffered cardiac arrest and was pronounced dead after being rushed to a local hospital.
An investigation by the Florida Department of Health concluded the procedure had “no substantiated medical or scientific value.”
Two years earlier, in 2010, 69-year old Domenica Fitzgerald suffered a stroke after Grekos infused unfiltered bone marrow-derived stem cells into the arteries of her brain. The state report concluded “it was virtually inevitable that the procedure would clog blood vessels in the brain and cause a major and very possibly fatal stroke.” Fitzgerald suffered severe brain damage and was later removed from life support.
Jack Fitzgerald says his wife, who used a wheel chair, had hoped that stem cells might help her walk again in time for her grandson’s college graduation. She had previously suffered nerve damage as a side effect of chemotherapy used to treat breast cancer.
“When they’re desperate, people try things,” Fitzgerald says. “When you’re really ill you’ll say, ‘Let me give it a shot and see if it can help me.’”
After Domenica Fitzgerald’s death, the state ordered Grekos to stop using stem cells in his practice, but he did not. Not until 2013, after Poling’s death, did the Florida Medical Board unanimously vote to revoke the doctor’s license.
“It was proved overwhelmingly that the procedures he used did not meet the standard of care,” says Natalie Spindle, spokeswoman for the Department of Health.
Fitzgerald and his family brought a wrongful death lawsuit against Grekos, which was settled out of court for an undisclosed amount. The Poling family considered suing Grekos, but their lawyer advised that little money was left to recover.
Both families had hoped the Florida state attorney general would bring criminal charges against Grekos. But last May, state prosecutors declined to move forward with the case.
Though barred from practicing medicine in Florida, Grekos continues to treat patients in the Dominican Republican through his company Regenocyte, which promotes treatments for autism, dementia, cystic fibrosis and many other diseases.
He believes the two patients’ deaths were unrelated to his care — the state targeted him to discourage other doctors from working with stem cells, he says.
MURKY REGULATION
The regulators tasked with weeding out dangerous medical practices are the 50 state medical boards responsible for licensing and disciplining health professionals. But those groups have taken action against only a handful of stem cell doctors.
Last year, the Oregon Medical Board revoked the license of Dr. Kenneth Welker, a Eugene physician who performed at least five experimental stem cell procedures. In one case, a 62-year-old woman who received a spinal injection of stem cells experienced tingling, elevated blood pressure and rapid breathing, according to the board’s complaint. The group fined Welker $10,000 and revoked his license, citing “unprofessional or dishonorable conduct.”
State sanctions against stem cell doctors are rare because medical boards generally begin investigating practitioners only after patients have been harmed. That’s led many industry critics to conclude that regulation must come from the FDA, which regulates experimental drugs and medical products on a national level.
But the FDA’s authority to regulate stem cell procedures is not clearly defined and has been debated by legal experts for years. In that time, the agency has cracked down only on a handful of clinics, which industry observers say has emboldened stem cell entrepreneurs.
Lee Buckler, a stem cell consultant for drug and device makers, says many doctors “believe that there is no problem with what they’re doing because the FDA hasn’t knocked on their door.”
The key issue in FDA’s oversight of cell-based medicines turns on how much processing the stem cells undergo. According to FDA regulations, human cells that are more than “minimally manipulated” are subject to the same regulations as prescription drugs. But “minimal manipulation” is not clearly defined in FDA guidelines.
When the FDA has shut down stem cell clinics, it has usually been for growing stem cells in the laboratory for weeks at a time. In at least two cases, the FDA said that that approach exceeds the “minimal manipulation” threshold and could endanger patients due to potential contamination and infection.
But the FDA appeared less interested in policing the far more common practice of same-day stem cell procedures, in which cells are extracted and returned to patients in a few hours.
Now, the FDA appears to be stepping up its oversight. In the last days of 2014, it released draft guidelines dealing with these procedures. The agency said that processing fat into stem cells for medical use is more than “minimal manipulation,” essentially creating a new drug, which cannot be sold in the U.S. without the agency’s approval.
The guidelines have not been finalized — a process that can take years. And the agency said in a statement that it hopes doctors will use the proposed rules to essentially police themselves “and reduce the need for enforcement actions.”
The FDA declined to make officials available for an on-the-record interview for this story. But in a statement, it said it “takes violations of the regulations seriously and routinely follows up on entities producing products that potentially violate FDA regulations.”
Many stem cell doctors continue to argue that they don’t need FDA permission because they are not creating drugs, but performing in-office surgical procedures, which are not regulated by the agency.
But Alta Charo, a professor of law and bioethics at University of Wisconsin, says the FDA’s draft guidelines make clear that processed fat stem cells meet the same definition as prescription drugs. “You cannot sell that in the United States without it having been approved,” says Charo, who spent two years at the FDA as a policy adviser.
Getting a drug approved by the FDA requires extensive clinical testing. And if stem cell doctors followed the FDA’s requirements, they would not be able to begin testing their techniques on patients until completing a rigorous FDA application process, which can take years and cost millions of dollars. Patients would then be carefully tracked through large clinical trials, measured against a control group of patients receiving a sham treatment or a traditional therapy.
Academic researchers are slowly moving ahead with hundreds of their own, more traditional studies. Researchers at the University of Florida, Duke University and six other universities are studying fat-derived stem cells as a treatment for heart disease. The trial is sponsored by Cytori Therapeutics, a company that makes a fat-processing stem cell device.
Elsewhere, doctors at the Mayo Clinic in Minnesota are investigating stem cells for Lou Gehrig’s Disease. And Baylor College of Medicine in Texas plans to begin enrolling patients for a study using stem cells to treat erectile dysfunction.
In the meantime, doctors who perform stem cell procedures continue to practice what they see as transformative medicine.
For now, Berman says he has no plans to change his business because he is helping people.
“How is it unethical if you’re actually helping people, even if we don’t have evidence-based studies to prove it?” he says.
He adds: “If I’m breaking the law, how come I haven’t been arrested yet?”
Copyright 2015 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.
by Topic
DISCLAIMER:
The information in these press releases is historical in nature, has not been updated, and is current only to the date indicated in the particular press release. This information may no longer be accurate and therefore you should not rely on the information contained in these press releases. To the extent permitted by law, RepliCel Life Sciences Inc. and its employees, agents and consultants exclude all liability for any loss or damage arising from the use of, or reliance on, any such information, whether or not caused by any negligent act or omission.
THIRD PARTY CONTENT
Please note that any opinion, estimates or forecasts made by the authors of these statements are theirs alone and do not represent opinions, forecasts or predictions of RepliCel Life Sciences Inc. or its management. RepliCel Life Sciences Inc. does not, by its reference or distribution of these links imply its endorsement of, or concurrence with, such information, conclusions or recommendations.